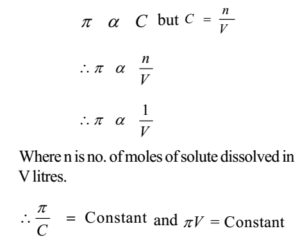Calculate the amount of CaCl2 (van't Hoff factor i = 2.47 ) dissolved in 2.5 L solution so that its osmotic pressure at 300K is 0.75 atmosphere.Given: Molar mass of CaCl2 is
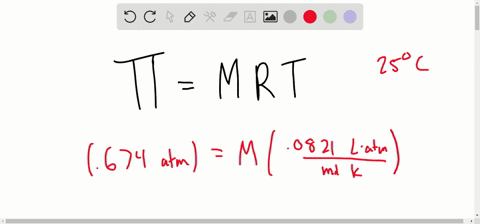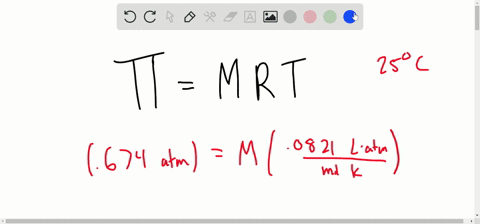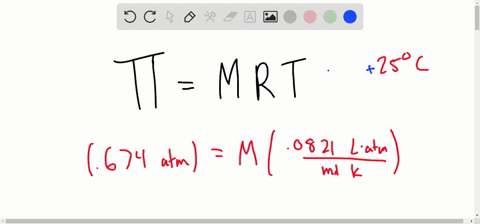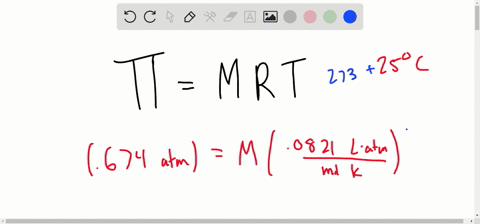
SOLVED:Determine the molarity of each of the following solutions from its osmotic pressure at 25^∘ C . Include the van 't Hoff factor for the solution when the factor is given. a.
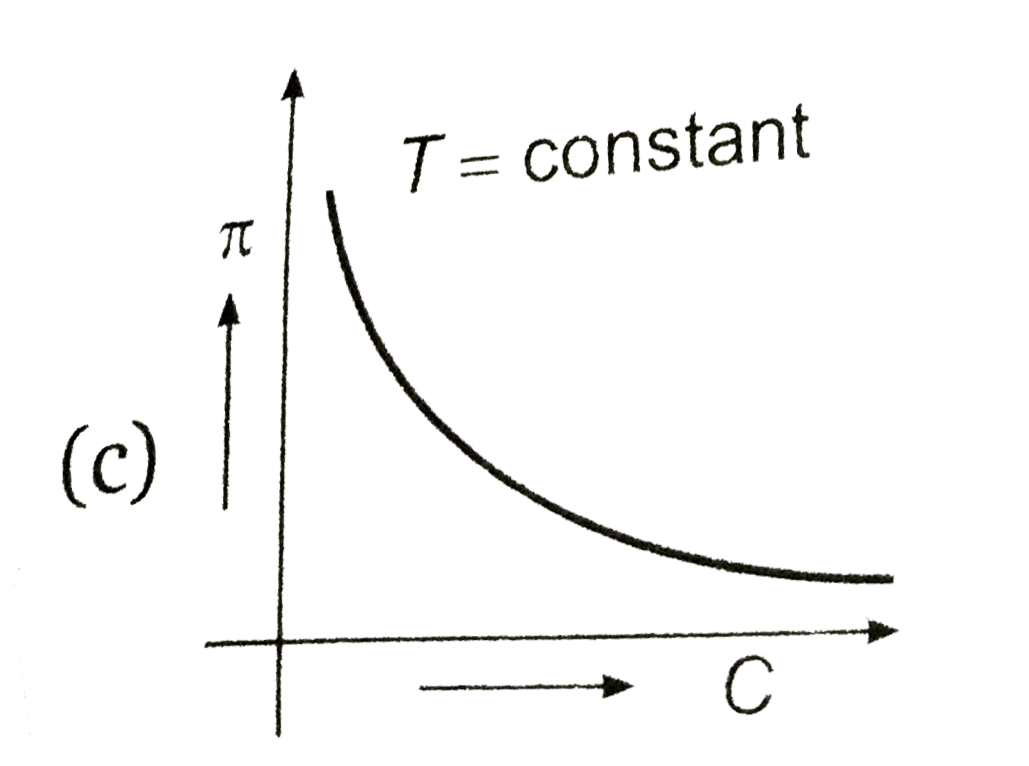
van't Hoff proved that osmotic pressure (pi) is a colligative property. For an ideal solution, osmotic pressure(pi) is helpful to determine that molecular mass of solute using M(B)=(W(B)RT)/(pi.V) Relation can exxpressed by

Osmosis/Osmotic Pressure/Vant Hoff Equation/Solutions/Explanation in Tamil/TN11th Std/CBSE 12 - YouTube

Calculate the amount of CaCl2 (van't Hoff factor i = 2.47 ) dissolved in 2.5 L solution so that its osmotic pressure at 300K is 0.75 atmosphere.Given: Molar mass of CaCl2 is

The observed osmotic pressure for a 0.10M solution of Fe (NH4)2 (SO4)2 at 25^o C is 10.8 atm. The expected and experimental (observed) values of Van't Hoff factor (i) will be respectively: (