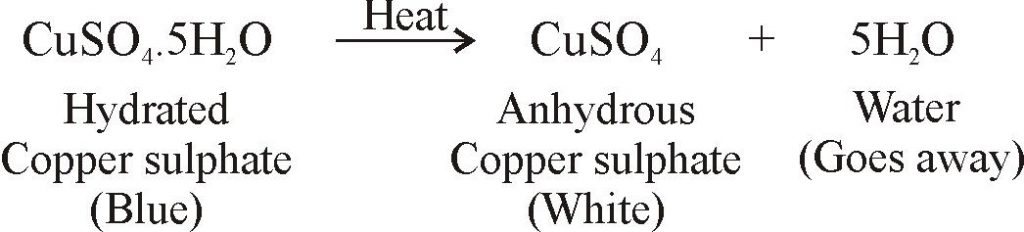
Question Video: Calculating the Percentage of Water of Crystallization Given the Mass of the Hydrated and Dehydrated Salt | Nagwa
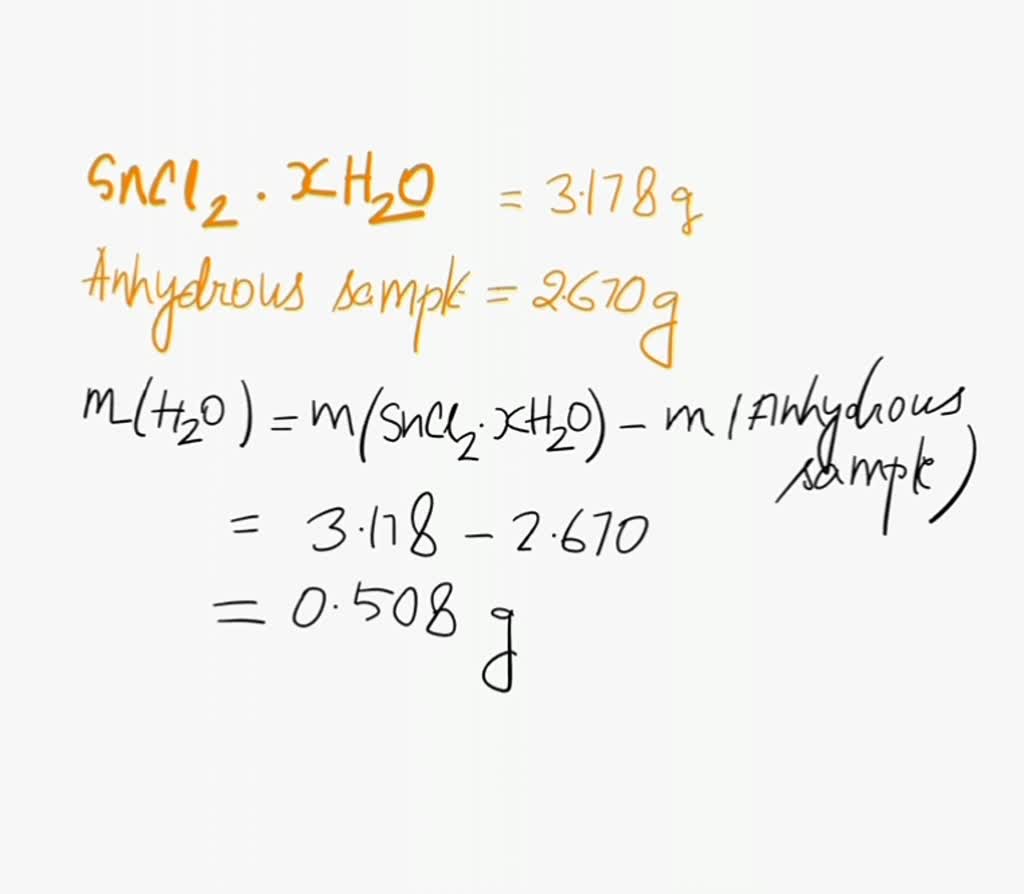
SOLVED: A student heated a sample of a hydrated salt of cobalt (II) chloride and obtained the following data: Grams of CoCl2 -X H2O used: 32.860 grams Grams of anhydrous CoCl2 (129.84

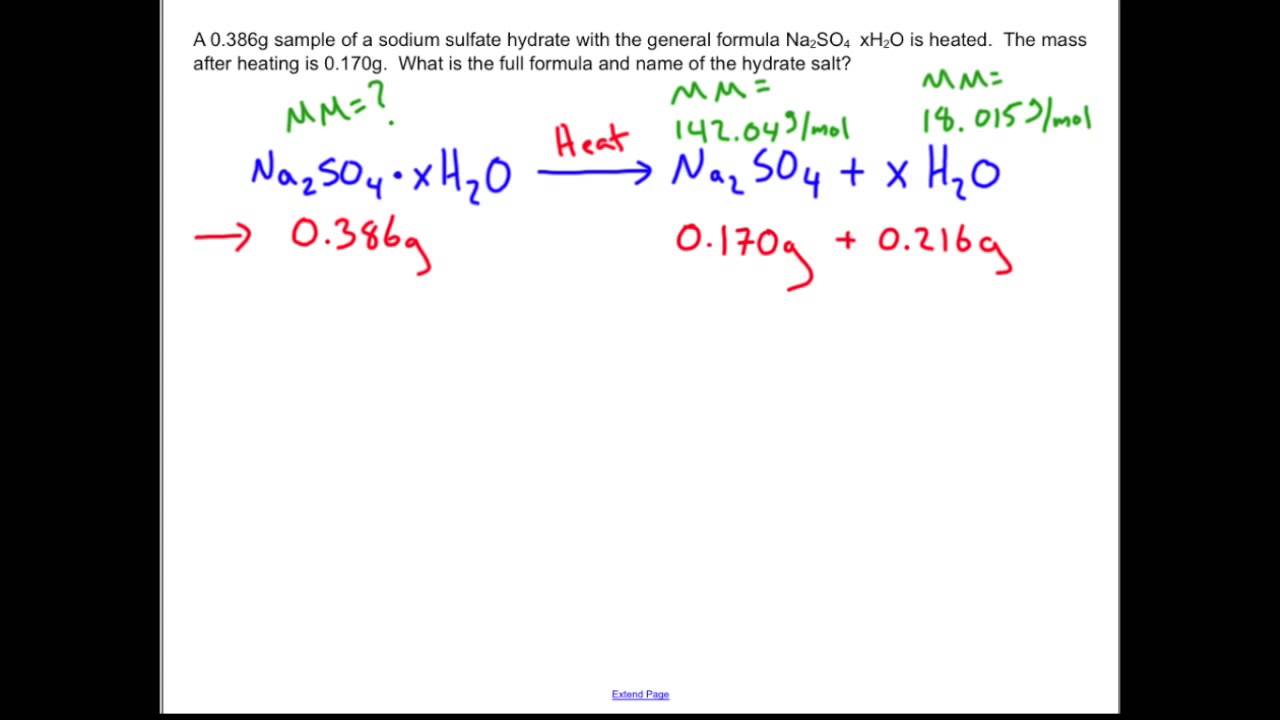
SOLVED: A1.16 g of hydrated sodium sulfate salt (Na2so4XH2O) was heated in a crucible for about 15 minutes to get 0.51g of the anhydrous salt (Mm of Na2so4 = 142.06 g/mol): Calculate: